File Name: types of electronic transitions in uv spectroscopy creator.zip
Size: 28820Kb
Published: 09.08.2021
In the present chapter, UV-Vis and Infrared spectroscopy have been discussed. Ultraviolet and Visible Spectroscopy This absorption spectroscopy uses electromagnetic radiations between 190 nm to 800 nm and is divided into the ultraviolet (UV, 190-400 nm) and visible (VIS, 400-800 nm) regions. Principles and applications of UV-visible spectroscopy The energy associated with electromagnetic radiation is defined by the following equation: where E is energy (in joules), h is Planck’s constant (6.62 × 10-34Js), and ν is frequency (in seconds). Wavelength and frequency Electromagnetic radiation can be considered a combination of.

Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state.
Uranium determination by UV-Vis spectrophotometry in organic matrix. Concentrations of uranium in the process samples provide essential information required for nuclear process monitoring. A large number of techniques have been developed to allow uranium determination, but each technique possesses some advantages and disadvantages and cannot be applied without difficulty to all samples.
Uv visible spectrophotometer principle pdf creator
Contents Introduction and principle Quantitative measurment and electronic Transitions Instrumentation and the experiment Applications Limitations. What is Spectroscopy?? Spectroscopyis the use of the absorption, emission, or scattering of electromagnetic radiation by matter to qualitatively or quantitatively study the matter or to study physical processes. The matter can be atoms, molecules, atomic or molecular ions, or solids. Types of spectroscopy Emission Spectroscopy: A method ofchemical analysisthat uses the intensity of light emitted from a flame or spark at a particular wavelength to determine the quantity of element in a sample.
Uv Visible Spectroscopy
Chapter 2 Instrumentation theory and techniques. Page 76 UV-visible spectra generally show only a few broad absorbance bands. Compared a count rate which is then output to a device such as a printer or computer monitor. The geometry of an evpv. Chapter 1 Principles and applications of UV-visible spectroscopy.
These metrics are regularly updated to reflect usage leading up to the last few days. Citations are the number of other articles citing this article, calculated by Crossref and updated daily. Find more information about Crossref citation counts. The Altmetric Attention Score is a quantitative measure of the attention that a research article has received online. Clicking on the donut icon will load a page at altmetric. Find more information on the Altmetric Attention Score and how the score is calculated.
Laser wavelengths ranging from ultra-violet through visible to near infra-red can be used for Raman spectroscopy. Typical examples include but are not limited to :. The choice of laser wavelength has an important impact on experimental capabilities:. Spatial resolution. For example, with a nm laser, and a 0. Thus, achievable spatial resolution is partially dependent on choice of laser. Ultra-violet UV lasers for Raman spectroscopy typically include laser wavelengths ranging from nm through to nm.
Uv visible spectrophotometer principle pdf creator. principle which states that Generally, the most probable transition uv vis absorption spectroscopy pdf is from. species in uv vis absorption spectroscopy pdf either solid or aqueous state.
UV-VIS Spectroscopy and Its Applications
За ее спиной ТРАНСТЕКСТ издал предсмертный оглушающий стон. Когда распался последний силиконовый чип, громадная раскаленная лава вырвалась наружу, пробив верхнюю крышку и выбросив на двадцать метров вверх тучу керамических осколков, и в то же мгновение насыщенный кислородом воздух шифровалки втянуло в образовавшийся вакуум. Сьюзан едва успела взбежать на верхнюю площадку лестницы и вцепиться в перила, когда ее ударил мощный порыв горячего ветра.
Нет сомнений, что человеческий мозг все же совершеннее самого быстродействующего компьютера в мире. В какую-то долю секунды сознание Беккера засекло очки в металлической оправе, обратилось к памяти в поисках аналога, нашло его и, подав сигнал тревоги, потребовало принять решение. Он отбросил бесполезный мотоцикл и пустился бежать со всех ног. К несчастью для Беккера, вместо неуклюжего такси Халохот обрел под ногами твердую почву. Спокойно подняв пистолет, он выстрелил.
Ну и. Но тебе там понравится. ГЛАВА 50 Фил Чатрукьян остановился в нескольких ярдах от корпуса ТРАНСТЕКСТА, там, где на полу белыми буквами было выведено: НИЖНИЕ ЭТАЖИ ШИФРОВАЛЬНОГО ОТДЕЛА ВХОД ТОЛЬКО ДЛЯ ЛИЦ СО СПЕЦИАЛЬНЫМ ДОПУСКОМ Чатрукьян отлично знал, что к этим лицам не принадлежит.
In our discussion in “Introduction to the Electromagnetic Spectrum and Spectroscopy” we have discussed the different wavelengths for ultraviolet and visible lights which range from 10 nm to 400nm and 400nm to 780 nm respectively. The following chapter discusses to a greater extent the principles involved in the utility of ultraviolet-visible spectroscopy (UV-Vis) and the Beer-Lambert law which is useful in quantitative analysis of samples.
As was seen in the chapter for the “Introduction to the Electromagnetic Spectrum and Spectroscopy”, the energy of the radiation can be calculated by the equation:
E = h . ν
Thus the energy of the radiation in the visible range is generally: 36 to 72 kcal/mole while that in the ultraviolet range goes as high as 143 kcal/mole. This energy irradiated on the molecules can result in changes in the electronic nature of the molecule i.e. changes between ground state and excited states of electrons within the system. As a result, UV-visible spectroscopy is also known as electronic spectroscopy.
Every time a molecule has a bond, the atoms in a bond have their atomic orbitals merged to form molecular orbitals which can be occupied by electrons of different energy levels. Ground state molecular orbitals can be excited to anti-bonding molecular orbitals.

The electrons in a molecule can be of one of three types: namely σ (single bond), π (multiple-bond), or non-bonding (n- caused by lone pairs). These electrons when imparted with energy in the form of light radiation get excited from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) and the resulting species is known as the excited state or anti-bonding state.
- σ-bond electrons have the lowest energy level and are the most stable electrons. These would require a lot of energy to be displaced to higher energy levels. As a result these electrons generally absorb light in the lower wavelengths of the ultraviolet light and these transitions are rare.
- π-bond electrons have much higher energy levels for the ground state. These electrons are therefore relatively unstable and can be excited more easily and would require lesser energy for excitation. These electrons would therefore absorb energy in the ultraviolet and visible light radiations.
- n-electrons or non-bonding electrons are generally electrons belonging to lone pairs of atoms. These are of higher energy levels than π-electrons and can be excited by ultraviolet and visible light as well.
Most of the absorption in the ultraviolet-visible spectroscopy occurs due to π-electron transitions or n-electron transitions. Each electronic state is well defined for a particular system i.e. a double bond in 2-butene would have a particular energy level for the π-electons which when absorbs a specific (or quantized) amount of energy would get excited to the π* energy level for the electrons.
The figure below shows the different transitions between the bonding and anti-bonding electronic states.
Different transitions between the bonding and anti-bonding electronic states when light energy is absorbed in UV-Visible Spectroscopy.
Uv-visible Spectroscopy Principle In Pdf Format
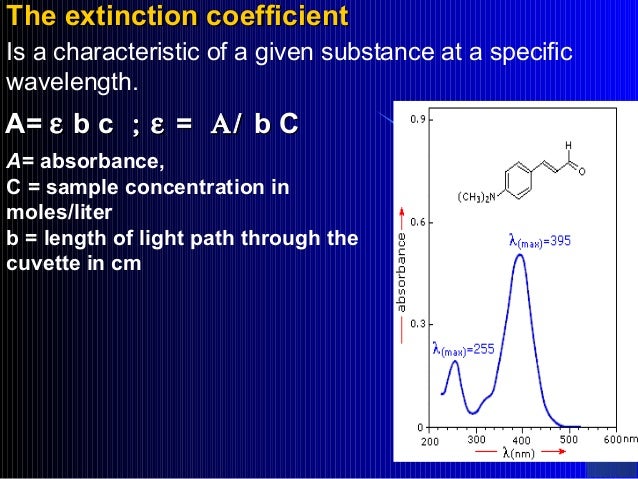
When a sample is exposed to light energy that matches the energy difference between a possible electronic transition within the molecule, a fraction of the light energy would be absorbed by the molecule and the electrons would be promoted to the higher energy state orbital. A spectrometer records the degree of absorption by a sample at different wavelengths and the resulting plot of absorbance (A) versus wavelength (λ) is known as a spectrum. The wavelength at which the sample absorbs the maximum amount of light is known as λmax. For example, shown below is the spectrum of isoprene. Isoprene is colorless as it does not absorb light in the visible spectrum, and has a λmax of 222nm.
UV-visible spectrum of isoprene showing maximum absorption at 222 nm.
Chromophore
Uv Vis Spectroscopy Principle Pdf
Certain chemical groups or entitities are susceptible to absorb light due to the electronic configuration of the electrons in the functional group. These groups are known as chromophores. For example, the table below lists commonly found chromophores and their estimated absorbances.
| Chromophore | Example | Excitation | λmax (nm) | Solvent |
| C = C | Ethene | π → π* | 171 | Hexanes |
| C = O | Ethanal | π → π* n → π* | 180 290 | Hexane |
| N = O | Nitromethane | π → π* n → π* | 200 275 | Hexane |
Effect of Conjugation
Conjugation of π-electrons affects the energy levels of the π-electrons. When two double bonds are conjugated, the electrons in them create four molecular orbitals (i.e. two bonding and two anti-bonding). See the figure below. As a result of this the highest occupied molecular orbital (HOMO) is at a higher energy state and the lowest unoccupied molecular orbital (LUMO) is of at a lower energy state. In order to excite this system, the energy that would be required to excite the electrons from the HOMO to the LUMO would therefore be reduced. As a result of this reduction in energy levels, the wavelength for absorption of conjugated molecules increases.
Diagram shows how a non-conjugated system would require more energy for absorption as compared to conjugated system.
Terminology for Absorption Shifts
| Nature of the Shift | Descriptive Term |
| To Greater Absorbance | Hyperchromic |
| To Lesser Absorbance | Hypochromic |
| To Longer Wavelength | Bathchromic or Red Shift |
| To Shorter Wavelength | Hypsochromic or Blue Shift |
Why Ultraviolet-Visible Spectra are not Sharp?
Uv-visible Spectroscopy Principle In Pdf Reader

Between the different electronic energy levels are the vibrational energy levels caused due to vibrational changes within the system. It must be remembered that UV-visible light can excite molecular vibrational levels as well. As a result of this phenomenon, there is not one sharp peak obtained in the UV-Visible spectra, but rather a smooth curve shaped peak for absorption as will be seen in several examples.
Vibrational energy levels cause ultraviolet-visible spectra to be smooth and not sharp peaks.
What Is The Principle Of Uv Visible Spectroscopy
Books on Analytical Chemistry and Spectroscopy
Uv-visible Spectroscopy Principle In Pdf Software
Check out these good books for analytical chemistry and spectroscopy
Amazon.com Widgets
Uv-visible Spectroscopy Principle In Pdf
- Analytical Chemistry: An Introduction (Saunders Golden Sunburst Series) 7th Ed., by Douglas A. Skoog, Donald M. West, F. James Holler. 1999.
- Fundamentals of Analytical Chemistry 8th Ed., by Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. 2003.